Description
Hieff NGSTM EvoMax RNA Library Prep Kit (dUTP) is a premixed, actinomycin D free and strand-specific total RNA sequencing library prep kit compatible with Illumina and MGI platforms. This product is available in two types: tube or sealing kit, and this kit is more convenient whether by Automated Liquid Handling Device or manual manipulation . The kit contains RNA fragmentation reagents, reverse transcription reagents, strand-specific ds-cDNA synthesis reagents, and library amplification reagents. It can be linked with the mRNA purification kit or the rRNA removal kit to do mRNA or lncRNA research. This product optimizes the reverse transcription module to obtain a high chain specificity library without actinomycin D, which ensures the safety of the experimenters to a greater extent. All reagents were subjected to strict quality control and functional validation to maximize the stability and repeatability of the library preparation.
Specifications
|
Cat.No. |
12340ES24/12340ES96/12340ES97/12340ES98 |
|
Size |
24 T / 96 T / 96 T (automation) / 96 T (plate) |
Components
|
Components No. |
Name |
12340ES24 |
12340ES96 |
12340ES97 (automation ) |
12340ES98 (Plate )** |
|
12340-A |
Frag/Prime Buffer |
450 μL |
2×900 μL |
2×1064 μL |
8×266 μL |
|
12340-B |
1st Reaction Module 2.0 |
192 μL |
768 μL |
960 μL |
8×120 μL |
|
12340-C |
2nd Reaction Module(dUTP) |
840 μL |
3×1120 μL |
3×1280 μL |
8×480 μL |
|
12340-D |
Ligation Reaction Module |
840 μL |
3×1120 μL |
3×1280 μL |
8×480 μL |
|
12340-E |
2×Super Canace® II High-Fidelity Mix |
600 μL |
2×1200 μL |
2×1360 μL |
8×340 μL |
|
* |
Primer Mix* |
/ |
/ |
/ |
/ |
Note: * The notation indicates that the component is not included in the kit. Primer mix is required if the complete adaptors are used, otherwise it isn’t. This kit is compatible with Illumina and MGI platforms, but need additional primer mix (Cat # 13334 Primer Mix for MGITM and Cat # 13335 Primer Mix for Illumina®) for Illumina® or MGI platform if the complete adaptors are used.
Figure
The layout of plate reagent group.

Figure 1: reagent layout of sealing-plate library kit

Figure 2. Simplied components of EvoMax RNA Library Prep Kit.
Storage
This product should be stored at -25~-15℃ for 1 years.
Instructions
1. The recommended amount to add in a 20 μL system is 0.1-1 units (U), and the input quantity can be adjusted based on the actual results.
2. According to the demands of the experiment, the final concentration of dUTP can be adjusted between 0.2~0.6 mM, and 0.2 mM dTTP can be added selectively.
3. The reaction time at 25~37℃ can be adjusted within 5~10 min according to the experimental require.
Notes
1. Operation
1) Please operate with lab coats and disposable gloves,for your safety.
2) Thaw components at room temperature. Mix thoroughly by inverting up and down several times, spin down briefly and place on ice for use.
3) It is recommended to perform each step reaction in a thermal cycler with a heated lid. The thermal cycler should be preheated to the set temperature prior to use.
4) Supplies free of RNase contamination and cleaning the experimental area regularly are necessary. ThermoFisher's RNAZapTM high-efficiency nucleic acid removal spray was recommended to remove RNase contamination.
5) Improper operations may very likely cause aerosol contaminations, impacting the accuracy of result. Mandatory physical isolation of PCR reaction mixing regions and PCR product purification assay regions is recommended. Equipped with equipment such as specialized pipettes for library construction.
6) This reagent is for one-time use. Multiple use is strictly prohibited.
7) This product is for research use only.
2. Adapter Ligation
1) Illumina or MGI Long Adapter (Barcoded Adapter) kits and short Adapter kits are available for customers to choose according to their experimental requirements.
2) Selecting high-quality, commercial adapters was recommended. If self-made adapters are selected, please entrust a company with experience in NGS primer synthesis and remark the need for strict contamination control. In addition, it is recommended to prepare DNA annealing solution in a clean bench and only operate one type of adapter each time to prevent cross-contamination.
3) Please thaw the adapters on the ice or at 4°C; when operating at room temperature, the laboratory temperature should not exceed 25°C to prevent the adapters from denaturing.
4) The concentration of the adapter directly affects the ligation efficiency and library yield. The adapter volume added to the kit is fixed to 5 μl. The adapters are recommended to be diluted with 0.1×TE buffer and the diluted adapters can be stored at 4°C for 48 hours. Table 1 lists the recommended adapter amount for different amounts of input RNA.
Table 1-1 The recommended Illumina adapter amount for different input RNA
|
Input Total RNA |
Illumina® Adapter stock concentration |
|
10 ng |
1 μM |
|
100 ng |
1.5 μM |
|
500 ng |
3 μM |
| ≥1 μg |
5 μM |
Table 1-2 The recommended MGI adapter amount for different input RNA
|
Input Total RNA |
MGI® Adapter stock concentration |
|
10~99 ng |
1 μM |
|
100~499 ng |
2 μM |
|
500~4000 ng |
5 μM |
* Adapter usage can be adjusted according to different types of total RNA samples and input amount
3. Library Amplification
1) On the basis of the first-generation DNA polymerase, the high-fidelity DNA polymerase in the kit has greatly improved its amplification uniformity and exhibits no amplification bias.
2) Amplification cycle numbers should be strictly controlled. Insufficient amplification may lead to low library yield; Over-amplification may introduce increased bias, errors, duplicated read, chimeric products and accumulation of expansion mutations. Table 2 lists the recommended cycle numbers for PCR amplification.
3) The recommended number of cycles recommended in Table 2 can meet the vast majority of library prep requirements. If your sample is of poor quality (such as FFPE samples with severe degradation), the number of cycles can be increased appropriately, according to the actual situation.Pay attention to note that mRNA varies from different species and tissues, the number of amplification cycles should be adjusted.
Table 2 Input Total RNA volume and amplification cycles recommended Table *
|
Input Total RNA |
Number of cycles |
|
10 ng |
16 |
|
100 ng |
14 |
|
500 ng |
12 |
|
1 μg |
11 |
[Note]: *The yield of the library is not only related to the input quantity and the number of amplification cycles, but also affected by the quality of samples, fragmentation conditions and sorting conditions. In the process of library construction, choose the most appropriate conditions according to the actual situation.
4. Bead-based DNA Cleanup and Size Selection
1) There are multiple steps in the library construction process that require DNA purification magnetic beads. We recommend Hieff NGS™ DNA Selection Beads (Yeasen Cat#12601) or AMPure® XP magnetic beads (Beckman Cat#A63880) for DNA purification and size-selection.
2) The magnetic bead should be balanced to room temperature before use, otherwise the yield will decrease and the size selecting effect will be affected.
3) The magnetic beads should be mixed well by vortex or pipetting prior to use.
4) Do not aspirate the beads when transferring the supernatant, even trace amounts of the beads may impact the following reactions.
5) The 80% ethanol should be freshly prepared, otherwise it will affect the recovery efficiency.
6) The magnetic beads should be dried at room temperature before eluting the product. Insufficient dryness will easily cause ethanol residual to affect subsequent reactions; excessive dryness will cause the magnetic beads to crack and reduce the purification yield. Normally, drying at room temperature for 3-5 minutes is enough to allow the beads to fully dry.
7) If needed, the purified or size-selected DNA samples eluted in TE buffer can be stored at 4°C for 1-2 weeks or at -20°C for a month.
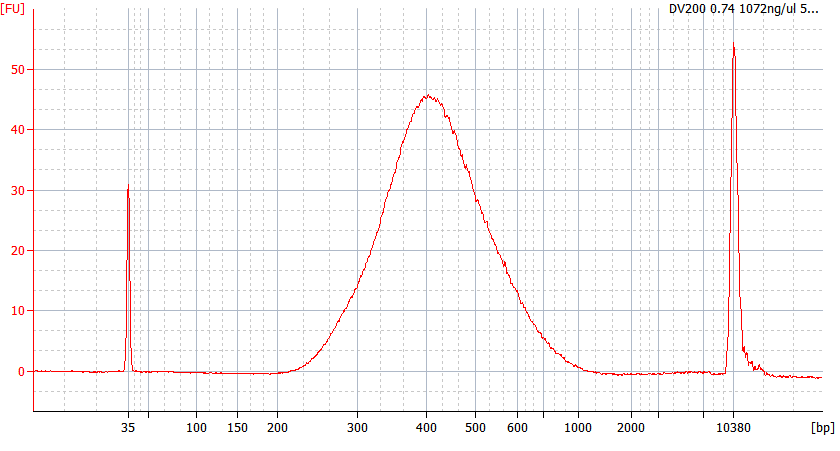
5. Library Quality Analysis
Generally, the quality of the constructed library can be evaluated by concentration detection and length distribution detection.
6. Other Material
1) mRNA enrichment kit: Hieff NGS® mRNA Isolation Master Kit V2 (Yeasen Cat # 12629).
2) rRNA removal kit: Hieff NGS® MaxUp Human rRNA Depletion Kit (rRNA & ITS / ETS) (Yeasen Cat # 12257) or other rRNA removal kit.
3) RNA purification of magnetic beads: Hieff NGS® RNA Cleaner (Yeasen Cat # 12602) or other equivalent products.
4) DNA purified magnetic beads: Hieff NGS® DNA Selection Beads (Yeasen Cat # 12601) or AMPure® XP Beads (A63880) or other equivalent products.
5) RNA quality control: Agilent 2100 Bioanalyzer RNA 6000 Nano / Pico Chip or other equivalent products.
6) Adapters: Complete Adapter for Illumina® use (Cat # 13519-13520 or other equivalent) or Complete Adapter for MGI® use (Cat # 13360-13362 or other equivalent).
7. Library quality inspection
Agilent 2100 Bioanalyzer DNA 1000 Chip / High Sensitivity Chip or other equivalent products; library quantification reagents.
8. Other materials
Anhydrous ethanol, sterile ultrapure water, low adsorption gun head, PCR tube, magnetic frame, PCR instrument, etc.
Flow chart

Figure 1: RNA library building process
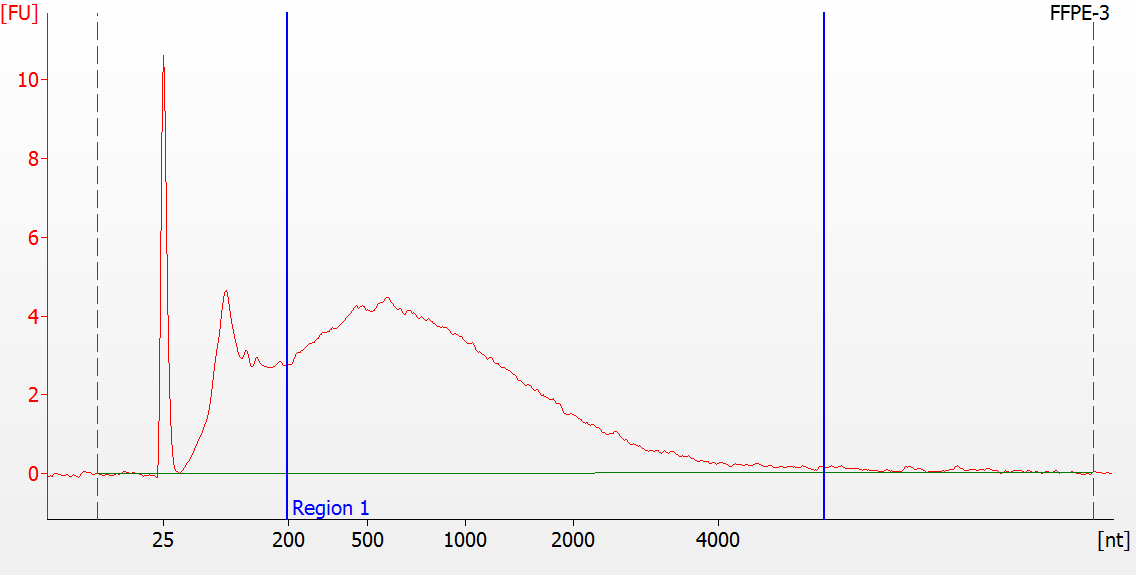
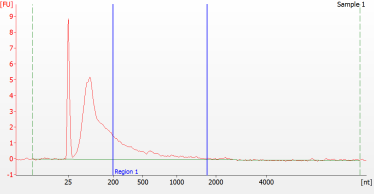
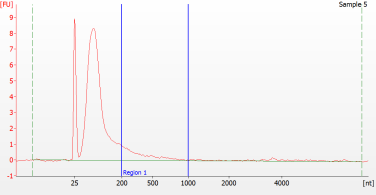
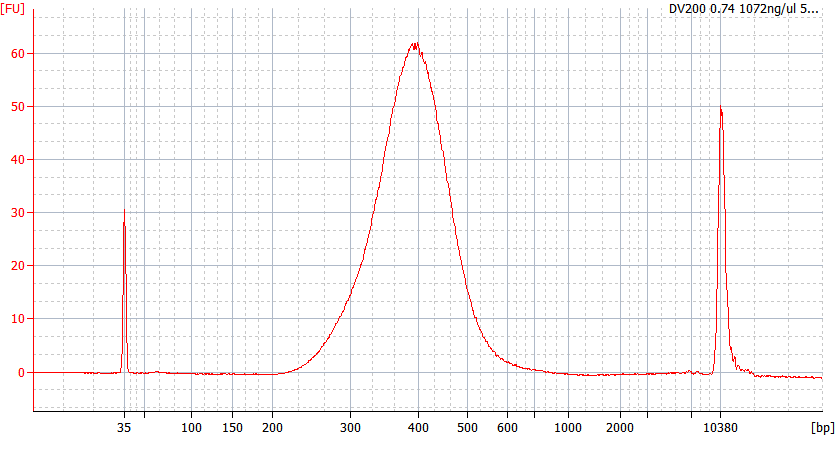
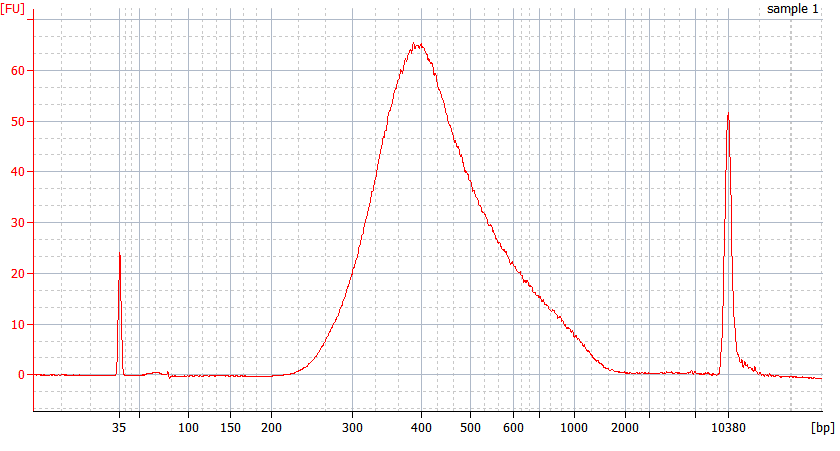
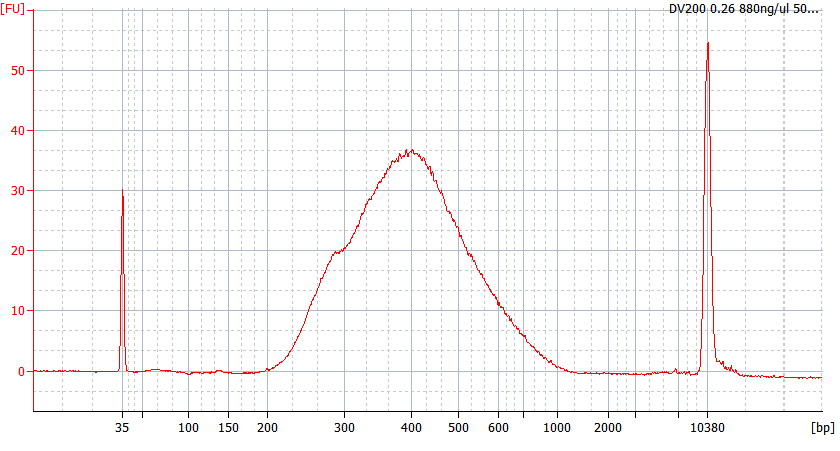
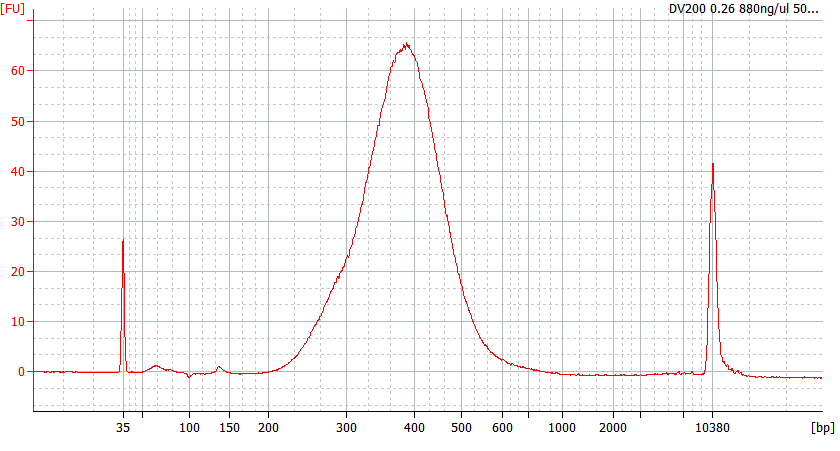
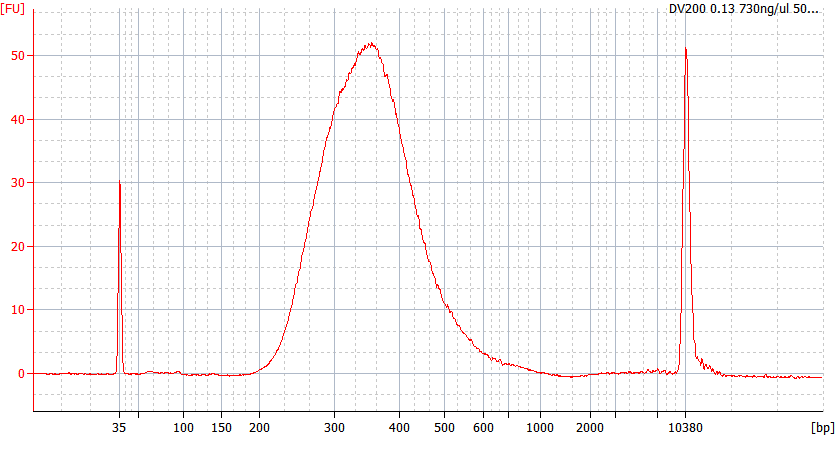
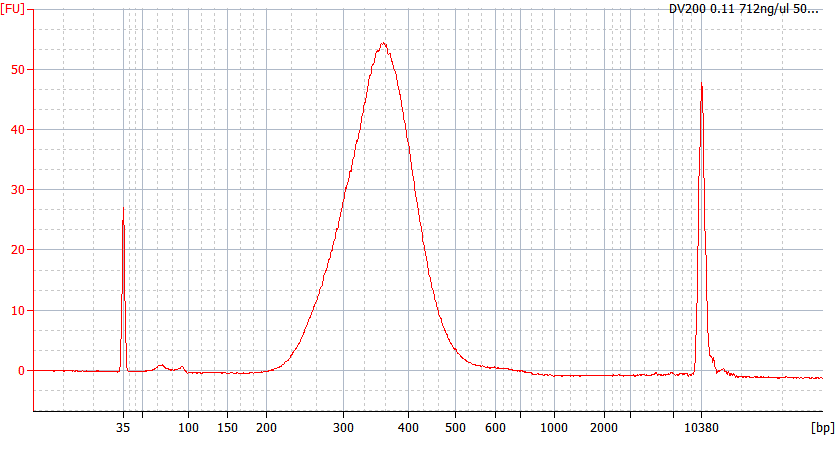
RNA Library prep for different quality FFPE samples
For severely degraded FFPE RNA (DV 200 <50%) and low input samples, we recommend a Double-purification protocol after adapter ligation to reduce library loss.
Peak patterns of different quality FFPE samples